Equation For Chlorine And Ammonia . Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. the reaction of ammonia with chlorine: — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. Ammonia reacts with chlorine to make chloramine (nh 2 cl),. In this chemical equation nh 3 is ammonia nd hocl. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2
from www.alamy.com
iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Ammonia reacts with chlorine to make chloramine (nh 2 cl),. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. the reaction of ammonia with chlorine: Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. In this chemical equation nh 3 is ammonia nd hocl. — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2
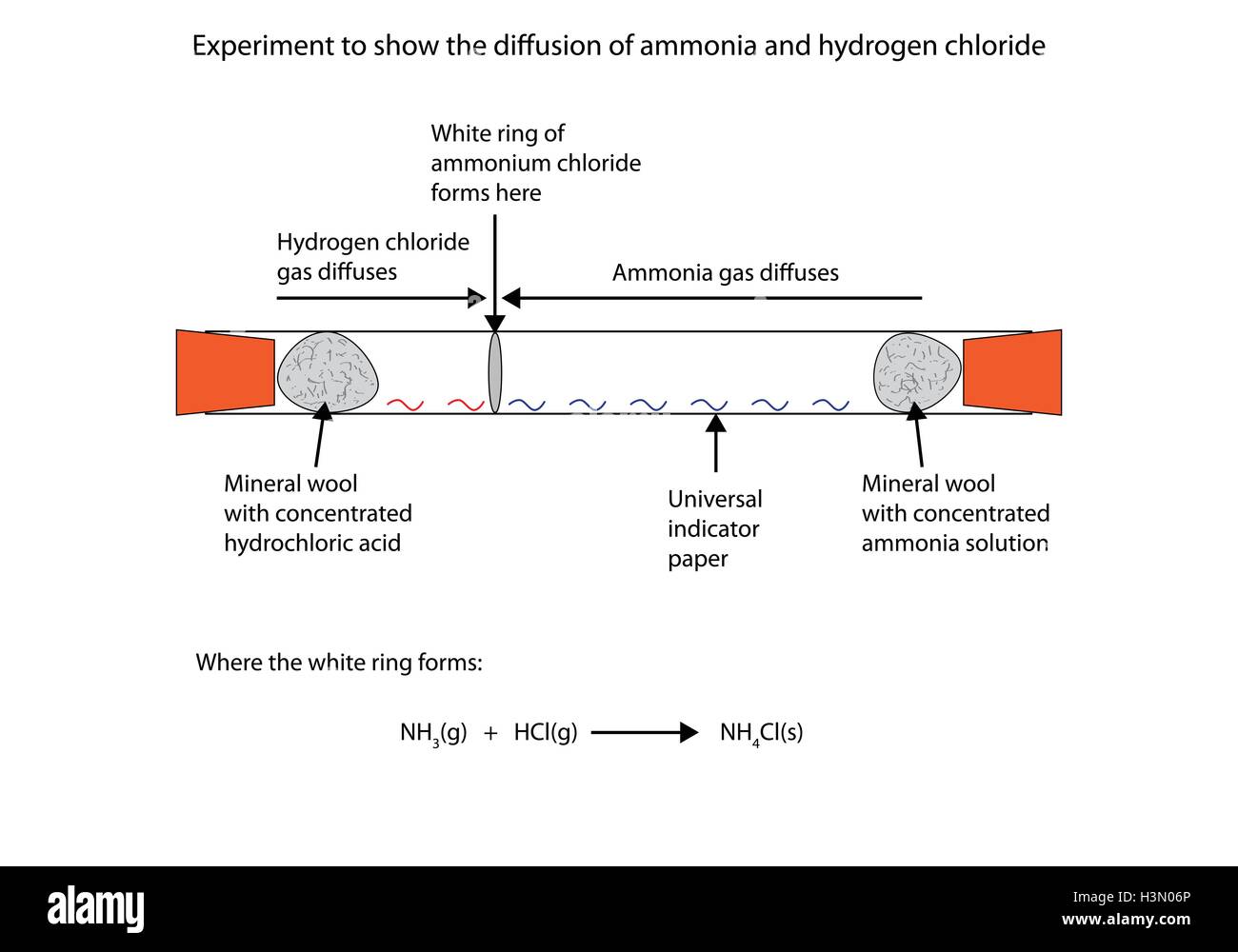
Labelled diagram to show the diffusion of ammonia and hydrogen Stock
Equation For Chlorine And Ammonia the reaction of ammonia with chlorine: In this chemical equation nh 3 is ammonia nd hocl. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2 — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. the reaction of ammonia with chlorine: give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. Ammonia reacts with chlorine to make chloramine (nh 2 cl),. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add.
From www.toppr.com
When excess of ammonia and chlorine react, nitrogen and ammonium Equation For Chlorine And Ammonia — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; the reaction of ammonia with chlorine: Ammonia reacts with chlorine to make chloramine (nh 2 cl),. In this chemical. Equation For Chlorine And Ammonia.
From brainly.in
balance the equation—Ammonia+Chlorine => Ammonium Chloride+NitrogenPlz Equation For Chlorine And Ammonia the reaction of ammonia with chlorine: — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. Ammonia reacts with chlorine to make chloramine (nh 2 cl),. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2 give the word equation and balanced molecular equation for the. Equation For Chlorine And Ammonia.
From www.youtube.com
How to Balance HCl + NH3 = NH4Cl (Hydrochloric acid + Ammonia) YouTube Equation For Chlorine And Ammonia the reaction of ammonia with chlorine: In this chemical equation nh 3 is ammonia nd hocl. Ammonia reacts with chlorine to make chloramine (nh 2 cl),. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride. Equation For Chlorine And Ammonia.
From cookinglove.com
Chlorine and ammonia Equation For Chlorine And Ammonia — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2 Ammonia reacts with chlorine to make chloramine (nh 2 cl),. Chlorine (cl. Equation For Chlorine And Ammonia.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Equation For Chlorine And Ammonia — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. Ammonia reacts with. Equation For Chlorine And Ammonia.
From www.numerade.com
SOLVED What are the two net ionic equations when aluminum nitrate Equation For Chlorine And Ammonia In this chemical equation nh 3 is ammonia nd hocl. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Ammonia reacts with chlorine to make chloramine (nh 2 cl),. the reaction of ammonia with chlorine: — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and. Equation For Chlorine And Ammonia.
From www.nagwa.com
Question Video Selecting the Correction Equation for the Reversible Equation For Chlorine And Ammonia iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. the reaction of ammonia. Equation For Chlorine And Ammonia.
From www.toppr.com
Write balanced chemical equations for each of the following (i) When Equation For Chlorine And Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl),. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. the reaction of ammonia with chlorine: 8nh 3 [excess] + 3cl 2 6nh 4. Equation For Chlorine And Ammonia.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Equation For Chlorine And Ammonia the reaction of ammonia with chlorine: iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2 give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium. Equation For Chlorine And Ammonia.
From www.essentialchemicalindustry.org
Chlorine Equation For Chlorine And Ammonia Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; In this chemical equation nh. Equation For Chlorine And Ammonia.
From cookinglove.com
Chlorine and ammonia Equation For Chlorine And Ammonia — bleach reacts with hydrochloric acid to produce chlorine gas, salt, and water. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; — the chemical. Equation For Chlorine And Ammonia.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Equation For Chlorine And Ammonia — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. 8nh 3 [excess] + 3cl 2 6nh 4 cl + n 2 Ammonia reacts with chlorine to make chloramine (nh 2 cl),. Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. the reaction. Equation For Chlorine And Ammonia.
From www.alamy.com
Fully labelled diagram of the laboratory preparation of hydrogen Stock Equation For Chlorine And Ammonia the reaction of ammonia with chlorine: — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride. Equation For Chlorine And Ammonia.
From www.tessshebaylo.com
Equation For Ammonia Reacting With Water Tessshebaylo Equation For Chlorine And Ammonia iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and. Equation For Chlorine And Ammonia.
From cookinglove.com
Chlorine and ammonia Equation For Chlorine And Ammonia — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; In this chemical equation nh 3 is ammonia nd hocl. — bleach reacts with hydrochloric acid to produce chlorine. Equation For Chlorine And Ammonia.
From dxosibdrh.blob.core.windows.net
Chlorine In Water Treatment Equation at Charles Patrick blog Equation For Chlorine And Ammonia Ammonia reacts with chlorine to make chloramine (nh 2 cl),. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; In this chemical equation nh 3 is ammonia. Equation For Chlorine And Ammonia.
From exogwagyf.blob.core.windows.net
Chlorine And Acid at Charles Borkowski blog Equation For Chlorine And Ammonia give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. iron(iii) oxide reacts with chlorine gas to give iron(iii) chloride and oxygen gas, according to the equation shown below; Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. — bleach. Equation For Chlorine And Ammonia.
From byjus.com
40. Write the chemical equations for the following reactions i Equation For Chlorine And Ammonia Chlorine (cl 2) gas reacts with ammonia (nh 3) gas giving different products based on. give the word equation and balanced molecular equation for the laboratory preparation of ammonia from nh 4 cl and calcium hydroxide. — the chemical feed rate equation above is used to determine the amounts of chlorine and ammonia to add. iron(iii) oxide. Equation For Chlorine And Ammonia.